Thank you very much for visiting the website of FS Lab Co., Ltd.
Our company was established in 2016, specializing in supporting the notification of functional foods and assisting with overseas health claims (ASEAN) applications. Since our establishment, we have been committed to contributing to society by enhancing the health and well-being of our customers, under the vision of “Changing society through the power of food.”
From 2024, we have been putting additional effort into supporting health claims applications for the ASEAN market. This allows us to assist not only within Japan but also in the international expansion of our clients’ products. By doing so, we actively support the recognition of our clients’ products in broader markets.
We strive to maximize the functionality of foods and provide support to appropriately convey their value. By facilitating the complex notification processes smoothly, we are dedicated to ensuring that our clients’ products reach a wider audience in the market.
Moving forward, FS Lab Co., Ltd. will continue to earn our clients’ trust and provide innovative services, contributing to the realization of a healthy and prosperous society.

President and CEO
Daigo Sato
●Advisor, Organization for Small & Medium Enterprises and Regional Innovation
●Advisory Committee Member, Korea Agro-Fisheries and Food Trade Corporation
●Kyushu Region Biocluster Promotion Council Advisor
●Director of the Japan Food Evidence Association
We have extensive knowledge of Japan’s functional food claims system. The Japanese system requires evidence based on the U.S. FDA regulations and international consensus. We conduct research on the necessary evidence by comparing Japan’s system with overseas systems.
We have provided consulting, academic research, and submission support for entering the Japanese functional food claims market to food ingredient manufacturers from the EU, South Korea, Singapore, Thailand, and China.

We will conduct a survey using an academic database to understand material properties. Based on the results of the research, we will decide on future development directions.
We will also conduct a regulation check simultaneously and prepare a report on any missing evidence and consistency with the system.

Based on the Feasibility Study & Regulation Check results, we will select various analyses. We will work with affiliated analysis institutions to carry out the necessary analyses.
If you require special analytical methods, we can also provide an original analytical environment.

We conduct “double-blind clinical trials (RCT)” as required by the Japanese functional food claims system. Additionally, if active results are produced, we undertake the writing and publication of academic papers.
In Japan’s functional food claims system, clinical trial results can be utilized as Form V.
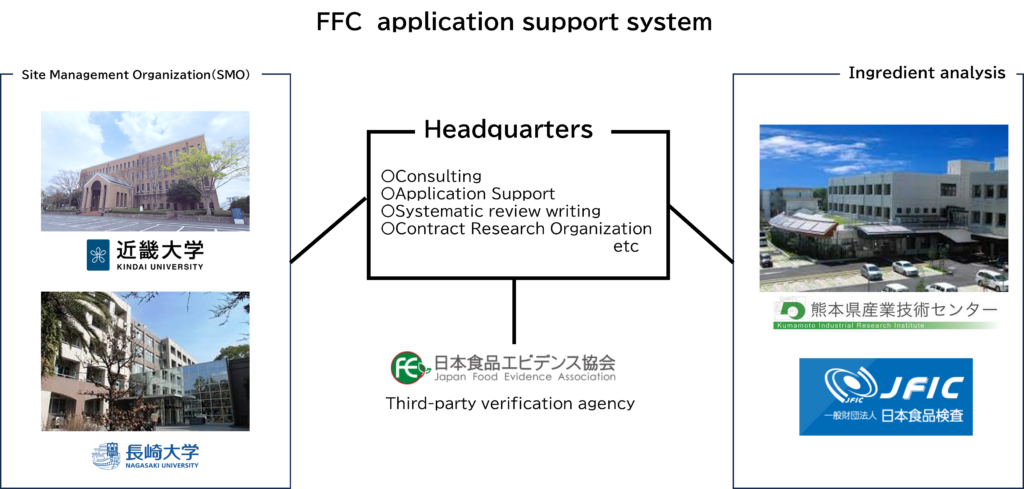
We prepare systematic reviews based on the global academic consensus “PRISMA 2020”.
The systematic reviews we prepare can be utilized as Form V in Japan’s functional food claims system.

We will prepare other necessary documents for the notification of functional food claims.
”Form Ⅰ” Consumer Information (Basic Information)
”Form Ⅱ” Safety Information
”Form Ⅲ” Manufacturing and Quality Control Information
”Form Ⅳ” Health Damage Collection System Information
”Form Ⅴ” Functionality Information / Mechanism of Action
”Form Ⅵ” Label Information
We support the development of sales channels for products with functional food claims in Japan through collaboration with our partner companies.
| Company name | FS-Lab Co., Ltd |
| CEO | DAIGO SATO |
| Address | Chiyo 4-4-16-2F Hakata-Ku Fukuoka-City 8120044 JAPAN |
| Capital | 1,000,000 JPY |
| Establish | 28/09/2016 |
| Phone | +81-92-203-6076 (Japanese only) |
| Our Business | Food CRO business Support for notification of foods with functional claims in Japan Support for applying for foods with overseas health claims |
ご要望・お問い合わせなど
お気軽にご相談ください
専任のスタッフがお客様のご不明な点にお答えいたします。
お困りでしたらお電話またはお問い合わせフォームからお気軽にお問い合わせください。
 エフエスラボ株式会社
エフエスラボ株式会社